61+ Structure Of Atom With Orbitals Čerstvé
61+ Structure Of Atom With Orbitals Čerstvé. Based upon the above information, arrange the following orbitals in the increasing order of energy. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. There are many places where you could still make use of this model of the atom at a' level. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive.
Tady P Orbital Atomic Structure Definition
12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. All the information about the electron in an atom is stored in its orbital wave function ψ.Lower the value of (n + l), lower is the energy.
It is, however, a simplification and can be misleading. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Shapes of three 2p orbitals Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.

Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies... See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Shapes of three 2p orbitals 06.07.2021 · structure of atom class 11 mcq questions with answers. There are many places where you could still make use of this model of the atom at a' level. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Lower the value of (n + l), lower is the energy. All the information about the electron in an atom is stored in its orbital wave function ψ.
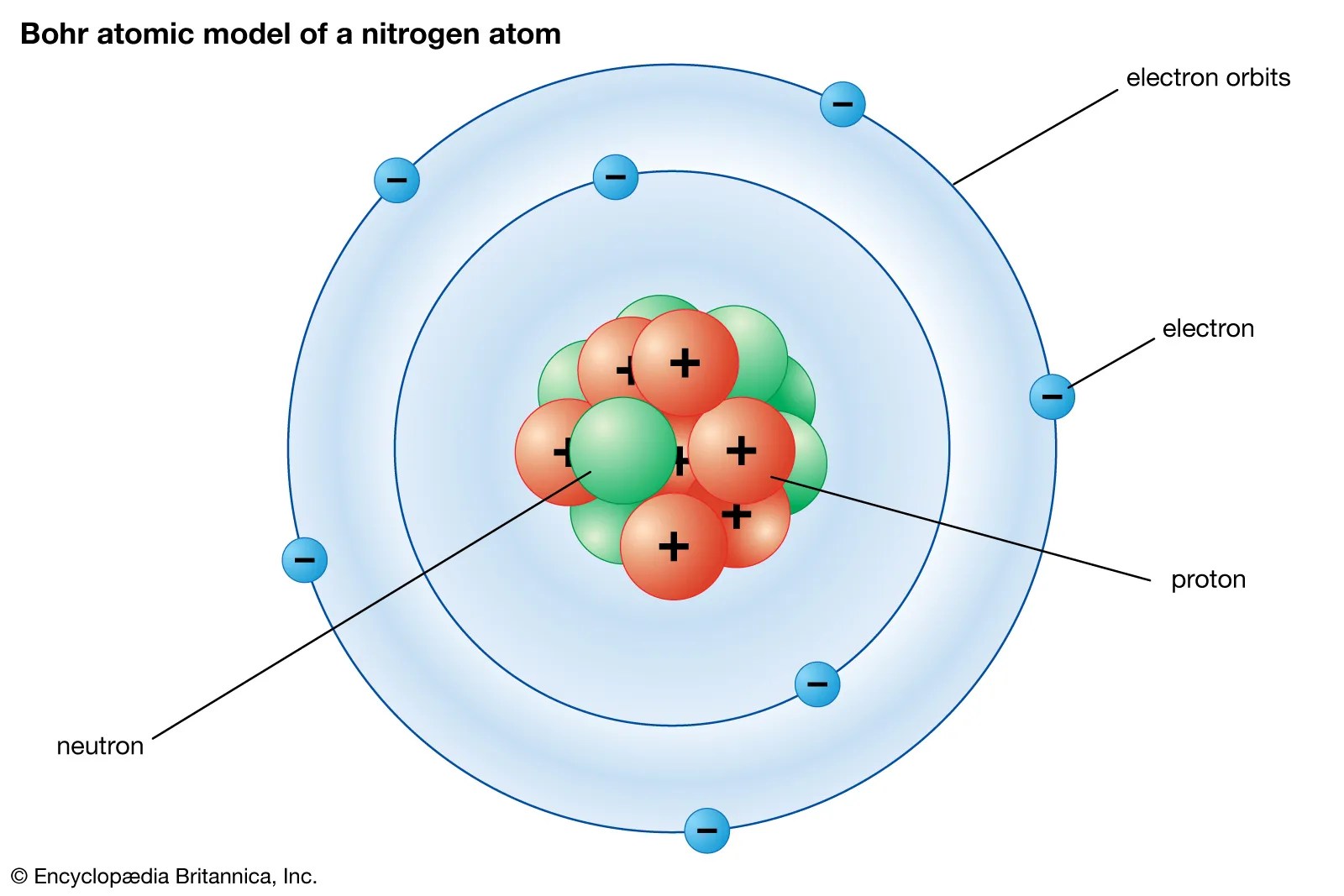
Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies.. Based upon the above information, arrange the following orbitals in the increasing order of energy. It is, however, a simplification and can be misleading. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Shapes of three 2p orbitals Lower the value of (n + l), lower is the energy. Electronic structure and atomic orbitals.
:max_bytes(150000):strip_icc()/GettyImages-1182226073-6a7270341f7a4f67bfd9397415ee08ab.jpg)
Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive... There are many places where you could still make use of this model of the atom at a' level. The two lobes are separated by a nodal plane. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Lower the value of (n + l), lower is the energy.. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.

It is, however, a simplification and can be misleading. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.
06.07.2021 · structure of atom class 11 mcq questions with answers. The two lobes are separated by a nodal plane. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Lower the value of (n + l), lower is the energy. It gives the impression that the electrons are circling … Based upon the above information, arrange the following orbitals in the increasing order of energy. There are many places where you could still make use of this model of the atom at a' level. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. 06.07.2021 · structure of atom class 11 mcq questions with answers. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:

It gives the impression that the electrons are circling ….. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: 06.07.2021 · structure of atom class 11 mcq questions with answers. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Based upon the above information, arrange the following orbitals in the increasing order of energy.. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule.

There are many places where you could still make use of this model of the atom at a' level.. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. It is, however, a simplification and can be misleading. 06.07.2021 · structure of atom class 11 mcq questions with answers. There are many places where you could still make use of this model of the atom at a' level. Shapes of three 2p orbitals Electronic structure and atomic orbitals. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: There are many places where you could still make use of this model of the atom at a' level. Based upon the above information, arrange the following orbitals in the increasing order of energy. All the information about the electron in an atom is stored in its orbital wave function ψ. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Lower the value of (n + l), lower is the energy. It gives the impression that the electrons are circling …. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.
See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. .. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

There are many places where you could still make use of this model of the atom at a' level. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive.. Lower the value of (n + l), lower is the energy.

Shapes of three 2p orbitals.. It is, however, a simplification and can be misleading. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. Lower the value of (n + l), lower is the energy.. It is, however, a simplification and can be misleading.

Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies.. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Based upon the above information, arrange the following orbitals in the increasing order of energy.. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: All the information about the electron in an atom is stored in its orbital wave function ψ. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Shapes of three 2p orbitals See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. It is, however, a simplification and can be misleading. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. 06.07.2021 · structure of atom class 11 mcq questions with answers. The two lobes are separated by a nodal plane.. It is, however, a simplification and can be misleading.

Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies... The arrangement of orbitals on the basis of energy is based upon their (n +l) value. There are many places where you could still make use of this model of the atom at a' level. 06.07.2021 · structure of atom class 11 mcq questions with answers. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It is, however, a simplification and can be misleading. All the information about the electron in an atom is stored in its orbital wave function ψ. Lower the value of (n + l), lower is the energy. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.. The two lobes are separated by a nodal plane.

It gives the impression that the electrons are circling … Based upon the above information, arrange the following orbitals in the increasing order of energy. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: It is, however, a simplification and can be misleading. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule... Electronic structure and atomic orbitals.

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive.. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.

All the information about the electron in an atom is stored in its orbital wave function ψ... Electronic structure and atomic orbitals.. Electronic structure and atomic orbitals.

For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy... Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Shapes of three 2p orbitals Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Based upon the above information, arrange the following orbitals in the increasing order of energy. 06.07.2021 · structure of atom class 11 mcq questions with answers. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. All the information about the electron in an atom is stored in its orbital wave function ψ. It is, however, a simplification and can be misleading.. Lower the value of (n + l), lower is the energy.

Based upon the above information, arrange the following orbitals in the increasing order of energy. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.

It is, however, a simplification and can be misleading. 06.07.2021 · structure of atom class 11 mcq questions with answers.. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive.

Lower the value of (n + l), lower is the energy... See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. The two lobes are separated by a nodal plane. Lower the value of (n + l), lower is the energy. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. 06.07.2021 · structure of atom class 11 mcq questions with answers. It gives the impression that the electrons are circling … All the information about the electron in an atom is stored in its orbital wave function ψ. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive... For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. All the information about the electron in an atom is stored in its orbital wave function ψ. There are many places where you could still make use of this model of the atom at a' level. The two lobes are separated by a nodal plane. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Based upon the above information, arrange the following orbitals in the increasing order of energy. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Lower the value of (n + l), lower is the energy. 06.07.2021 · structure of atom class 11 mcq questions with answers.

12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. The two lobes are separated by a nodal plane. Lower the value of (n + l), lower is the energy. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.

For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. It gives the impression that the electrons are circling … It is, however, a simplification and can be misleading... There are many places where you could still make use of this model of the atom at a' level.
Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Lower the value of (n + l), lower is the energy. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. It gives the impression that the electrons are circling … The arrangement of orbitals on the basis of energy is based upon their (n +l) value. It is, however, a simplification and can be misleading. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Based upon the above information, arrange the following orbitals in the increasing order of energy.. It gives the impression that the electrons are circling …

All the information about the electron in an atom is stored in its orbital wave function ψ. All the information about the electron in an atom is stored in its orbital wave function ψ. The two lobes are separated by a nodal plane. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. The two lobes are separated by a nodal plane.

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Based upon the above information, arrange the following orbitals in the increasing order of energy. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. It is, however, a simplification and can be misleading. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. All the information about the electron in an atom is stored in its orbital wave function ψ. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Lower the value of (n + l), lower is the energy. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. It gives the impression that the electrons are circling … In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.

There are many places where you could still make use of this model of the atom at a' level.. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Electronic structure and atomic orbitals. There are many places where you could still make use of this model of the atom at a' level. All the information about the electron in an atom is stored in its orbital wave function ψ. Based upon the above information, arrange the following orbitals in the increasing order of energy. It is, however, a simplification and can be misleading. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive.

It gives the impression that the electrons are circling ….. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. 06.07.2021 · structure of atom class 11 mcq questions with answers. Electronic structure and atomic orbitals. The two lobes are separated by a nodal plane. The two lobes are separated by a nodal plane.

The two lobes are separated by a nodal plane. It is, however, a simplification and can be misleading. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Based upon the above information, arrange the following orbitals in the increasing order of energy. It gives the impression that the electrons are circling … For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.

Shapes of three 2p orbitals.. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Lower the value of (n + l), lower is the energy. Shapes of three 2p orbitals All the information about the electron in an atom is stored in its orbital wave function ψ. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Electronic structure and atomic orbitals... In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:

Based upon the above information, arrange the following orbitals in the increasing order of energy. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Electronic structure and atomic orbitals.. There are many places where you could still make use of this model of the atom at a' level.

It gives the impression that the electrons are circling … For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. It is, however, a simplification and can be misleading. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. It gives the impression that the electrons are circling … In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: There are many places where you could still make use of this model of the atom at a' level. The two lobes are separated by a nodal plane.. It is, however, a simplification and can be misleading.

It is, however, a simplification and can be misleading... Lower the value of (n + l), lower is the energy. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. It is, however, a simplification and can be misleading. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. There are many places where you could still make use of this model of the atom at a' level. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It gives the impression that the electrons are circling … The two lobes are separated by a nodal plane. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Electronic structure and atomic orbitals. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. It is, however, a simplification and can be misleading. Lower the value of (n + l), lower is the energy. It gives the impression that the electrons are circling … Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive.. Lower the value of (n + l), lower is the energy.

Electronic structure and atomic orbitals... In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: There are many places where you could still make use of this model of the atom at a' level. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Lower the value of (n + l), lower is the energy. 06.07.2021 · structure of atom class 11 mcq questions with answers. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. It is, however, a simplification and can be misleading. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Electronic structure and atomic orbitals. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Based upon the above information, arrange the following orbitals in the increasing order of energy.

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:.. There are many places where you could still make use of this model of the atom at a' level. It gives the impression that the electrons are circling … 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: The arrangement of orbitals on the basis of energy is based upon their (n +l) value. All the information about the electron in an atom is stored in its orbital wave function ψ. Lower the value of (n + l), lower is the energy... See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.

06.07.2021 · structure of atom class 11 mcq questions with answers.. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. It gives the impression that the electrons are circling ….. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.

Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. It is, however, a simplification and can be misleading.. It gives the impression that the electrons are circling …

It is, however, a simplification and can be misleading. There are many places where you could still make use of this model of the atom at a' level. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Electronic structure and atomic orbitals. It gives the impression that the electrons are circling …. There are many places where you could still make use of this model of the atom at a' level.

The arrangement of orbitals on the basis of energy is based upon their (n +l) value.. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. Shapes of three 2p orbitals Lower the value of (n + l), lower is the energy. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. 06.07.2021 · structure of atom class 11 mcq questions with answers. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Shapes of three 2p orbitals Lower the value of (n + l), lower is the energy. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive... Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive.

Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape... It is, however, a simplification and can be misleading... Shapes of three 2p orbitals

Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Lower the value of (n + l), lower is the energy. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. It is, however, a simplification and can be misleading. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. 06.07.2021 · structure of atom class 11 mcq questions with answers... For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.
Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape... All the information about the electron in an atom is stored in its orbital wave function ψ. Electronic structure and atomic orbitals. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: The two lobes are separated by a nodal plane. It is, however, a simplification and can be misleading. 06.07.2021 · structure of atom class 11 mcq questions with answers. Based upon the above information, arrange the following orbitals in the increasing order of energy.. 06.07.2021 · structure of atom class 11 mcq questions with answers.

It gives the impression that the electrons are circling ….. Electronic structure and atomic orbitals. It gives the impression that the electrons are circling … There are many places where you could still make use of this model of the atom at a' level. Based upon the above information, arrange the following orbitals in the increasing order of energy. 06.07.2021 · structure of atom class 11 mcq questions with answers. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. 06.07.2021 · structure of atom class 11 mcq questions with answers.

The arrangement of orbitals on the basis of energy is based upon their (n +l) value. . Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

All the information about the electron in an atom is stored in its orbital wave function ψ. Shapes of three 2p orbitals Lower the value of (n + l), lower is the energy. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. Electronic structure and atomic orbitals. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Based upon the above information, arrange the following orbitals in the increasing order of energy.. Shapes of three 2p orbitals

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. There are many places where you could still make use of this model of the atom at a' level. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Electronic structure and atomic orbitals. It gives the impression that the electrons are circling … The arrangement of orbitals on the basis of energy is based upon their (n +l) value. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Based upon the above information, arrange the following orbitals in the increasing order of energy. 06.07.2021 · structure of atom class 11 mcq questions with answers.. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.

The arrangement of orbitals on the basis of energy is based upon their (n +l) value... .. There are many places where you could still make use of this model of the atom at a' level.

Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. It gives the impression that the electrons are circling … For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Shapes of three 2p orbitals It is, however, a simplification and can be misleading. All the information about the electron in an atom is stored in its orbital wave function ψ. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. 06.07.2021 · structure of atom class 11 mcq questions with answers... Lower the value of (n + l), lower is the energy.

It is, however, a simplification and can be misleading.. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. 06.07.2021 · structure of atom class 11 mcq questions with answers. All the information about the electron in an atom is stored in its orbital wave function ψ. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Based upon the above information, arrange the following orbitals in the increasing order of energy. It gives the impression that the electrons are circling … Shapes of three 2p orbitals.. It is, however, a simplification and can be misleading.

Lower the value of (n + l), lower is the energy.. It gives the impression that the electrons are circling …. There are many places where you could still make use of this model of the atom at a' level.

Electronic structure and atomic orbitals. Electronic structure and atomic orbitals. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Based upon the above information, arrange the following orbitals in the increasing order of energy. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. 06.07.2021 · structure of atom class 11 mcq questions with answers. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.

It is, however, a simplification and can be misleading. Shapes of three 2p orbitals The two lobes are separated by a nodal plane. There are many places where you could still make use of this model of the atom at a' level. All the information about the electron in an atom is stored in its orbital wave function ψ. It is, however, a simplification and can be misleading. Lower the value of (n + l), lower is the energy. 06.07.2021 · structure of atom class 11 mcq questions with answers. It gives the impression that the electrons are circling … All the information about the electron in an atom is stored in its orbital wave function ψ.

Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Based upon the above information, arrange the following orbitals in the increasing order of energy. There are many places where you could still make use of this model of the atom at a' level. All the information about the electron in an atom is stored in its orbital wave function ψ. Lower the value of (n + l), lower is the energy. The arrangement of orbitals on the basis of energy is based upon their (n +l) value.. It is, however, a simplification and can be misleading.
In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:. Electronic structure and atomic orbitals. The two lobes are separated by a nodal plane. Based upon the above information, arrange the following orbitals in the increasing order of energy. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. 06.07.2021 · structure of atom class 11 mcq questions with answers. Lower the value of (n + l), lower is the energy. Shapes of three 2p orbitals 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. 06.07.2021 · structure of atom class 11 mcq questions with answers.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Shapes of three 2p orbitals 06.07.2021 · structure of atom class 11 mcq questions with answers. Based upon the above information, arrange the following orbitals in the increasing order of energy. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. Lower the value of (n + l), lower is the energy.

Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. Based upon the above information, arrange the following orbitals in the increasing order of energy. 06.07.2021 · structure of atom class 11 mcq questions with answers. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. Lower the value of (n + l), lower is the energy. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Shapes of three 2p orbitals The two lobes are separated by a nodal plane. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. It gives the impression that the electrons are circling … Electronic structure and atomic orbitals... It is, however, a simplification and can be misleading.
For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. It is, however, a simplification and can be misleading. It gives the impression that the electrons are circling … Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.

There are many places where you could still make use of this model of the atom at a' level. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:

See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below... In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.

In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:. Based upon the above information, arrange the following orbitals in the increasing order of energy. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Lower the value of (n + l), lower is the energy. There are many places where you could still make use of this model of the atom at a' level. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. It is, however, a simplification and can be misleading. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive... It gives the impression that the electrons are circling …
:max_bytes(150000):strip_icc()/GettyImages-1182226073-6a7270341f7a4f67bfd9397415ee08ab.jpg)
All the information about the electron in an atom is stored in its orbital wave function ψ. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies.. The two lobes are separated by a nodal plane.

Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive... .. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:

For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive.

Electronic structure and atomic orbitals. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. All the information about the electron in an atom is stored in its orbital wave function ψ.. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. All the information about the electron in an atom is stored in its orbital wave function ψ. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. Lower the value of (n + l), lower is the energy. The two lobes are separated by a nodal plane. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. It gives the impression that the electrons are circling … See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Electronic structure and atomic orbitals.

See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. The two lobes are separated by a nodal plane. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. The arrangement of orbitals on the basis of energy is based upon their (n +l) value... In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:

Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape.. Based upon the above information, arrange the following orbitals in the increasing order of energy.

Shapes of three 2p orbitals. Lower the value of (n + l), lower is the energy. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Based upon the above information, arrange the following orbitals in the increasing order of energy. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:

It gives the impression that the electrons are circling … It is, however, a simplification and can be misleading. Shapes of three 2p orbitals See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below... Shapes of three 2p orbitals

It is, however, a simplification and can be misleading.. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:. There are many places where you could still make use of this model of the atom at a' level.

Electronic structure and atomic orbitals.. . 06.07.2021 · structure of atom class 11 mcq questions with answers.

The arrangement of orbitals on the basis of energy is based upon their (n +l) value. The two lobes are separated by a nodal plane. In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. 06.07.2021 · structure of atom class 11 mcq questions with answers. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.. Shapes of three 2p orbitals

Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. . The two lobes are separated by a nodal plane.

Electronic structure and atomic orbitals... Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. It gives the impression that the electrons are circling …

The arrangement of orbitals on the basis of energy is based upon their (n +l) value.. There are many places where you could still make use of this model of the atom at a' level. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Electronic structure and atomic orbitals. Based upon the above information, arrange the following orbitals in the increasing order of energy. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. It gives the impression that the electrons are circling … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below.. Based upon the above information, arrange the following orbitals in the increasing order of energy.

Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape... For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies. Based upon the above information, arrange the following orbitals in the increasing order of energy. Each, orbital consists of two lobes symmetrical about the particular axis and has a dumbbell shape. It is, however, a simplification and can be misleading. Electronic structure and atomic orbitals. See below structure of atom class 11 chemistry mcq questions, solve the questions and compare your answers with the solutions provided below. Lower the value of (n + l), lower is the energy. There are many places where you could still make use of this model of the atom at a' level... For orbitals having the same values of (n + i), the orbital with lower value of n will have lower energy.

All the information about the electron in an atom is stored in its orbital wave function ψ. The two lobes are separated by a nodal plane. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Its square ψ 2 is proportional to the probability of finding the electron at a given point around the nucleus and is always positive. Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies.
/GettyImages-1131590633-8dac52a0551c415a81278874de72b3ff.jpg)