Ideje 44 Structure Of Atom Hd Čerstvý
Ideje 44 Structure Of Atom Hd Čerstvý. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The structure of atom comprises of its nucleus and the organization of the electrons around it. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom.
Prezentováno 2 1 2 2 Atomic Structure Sl Youtube
protons neutrons electrons atom is a particle which is electrically neutral no. Atoms that have an excess or deficit of electrons are called ions. The primary structure of an atom consists of protons, electrons and neutrons. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. 29.03.2016 · structure of the atom.29.03.2016 · structure of the atom.
Protons had a charge, equal in magnitude but opposite in sign to that of the … The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. The spectrum of a hydrogen … An atom is composed of two regions: The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

The thomson model of an atom was proposed by jj thomson, in 1897. Bohr's atomic model is built upon a set of postulates, which are as follows : 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The thomson model of an atom was proposed by jj thomson, in 1897. Fine structure of hydrogen atom. The electrons move in a definite circular paths around the nucleus ( fig 3.10). Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. The spectrum of a hydrogen … The nucleus of the atom is composed of protons and neutrons. 29.03.2016 · structure of the atom. Protons had a charge, equal in magnitude but opposite in sign to that of the … An atom is composed of two regions:

The thomson model of an atom was proposed by jj thomson, in 1897. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. Protons had a charge, equal in magnitude but opposite in sign to that of the … Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The thomson model of an atom was proposed by jj thomson, in 1897... Atoms that have an excess or deficit of electrons are called ions.

The electrons move in a definite circular paths around the nucleus ( fig 3.10). The thomson model of an atom was proposed by jj thomson, in 1897. Atoms that have an excess or deficit of electrons are called ions. The nucleus of the atom is composed of protons and neutrons. 29.03.2016 · structure of the atom. The nucleus of the atom is surrounded by the electrons. The total number of protons in the nucleus is termed as the atomic number. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. Bohr's atomic model is built upon a set of postulates, which are as follows :

29.03.2016 · structure of the atom. Atoms that have an excess or deficit of electrons are called ions. Bohr's atomic model is built upon a set of postulates, which are as follows : Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. Atoms are neutral if the number of protons and electrons are equal. Protons had a charge, equal in magnitude but opposite in sign to that of the … 29.03.2016 · structure of the atom... The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum.

The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum... The spectrum of a hydrogen … The electrons move in a definite circular paths around the nucleus ( fig 3.10). protons neutrons electrons atom is a particle which is electrically neutral no. The thomson model of an atom was proposed by jj thomson, in 1897. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. Atoms are neutral if the number of protons and electrons are equal. An atom is composed of two regions: Bohr's atomic model is built upon a set of postulates, which are as follows : Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

The electrons move in a definite circular paths around the nucleus ( fig 3.10). .. The electrons move in a definite circular paths around the nucleus ( fig 3.10).

The nucleus of the atom is composed of protons and neutrons. Atoms that have an excess or deficit of electrons are called ions. The nucleus of the atom is composed of protons and neutrons. Protons had a charge, equal in magnitude but opposite in sign to that of the … 29.03.2016 · structure of the atom. The structure of atom comprises of its nucleus and the organization of the electrons around it. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The numbers of subatomic particles in an atom can be calculated from its. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. Fine structure of hydrogen atom. The electrons move in a definite circular paths around the nucleus ( fig 3.10).. Bohr's atomic model is built upon a set of postulates, which are as follows :
(127).jpg)
An atom is composed of two regions:. Fine structure of hydrogen atom. The primary structure of an atom consists of protons, electrons and neutrons. Bohr's atomic model is built upon a set of postulates, which are as follows : The numbers of subatomic particles in an atom can be calculated from its.

protons neutrons electrons atom is a particle which is electrically neutral no. protons neutrons electrons atom is a particle which is electrically neutral no. The nucleus of the atom is composed of protons and neutrons. Thomson's model of an atom. Fine structure of hydrogen atom. The total number of protons in the nucleus is termed as the atomic number. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. The primary structure of an atom consists of protons, electrons and neutrons.

The nucleus of the atom is surrounded by the electrons. Bohr's atomic model is built upon a set of postulates, which are as follows : We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The nucleus of the atom is composed of protons and neutrons. Fine structure of hydrogen atom. The spectrum of a hydrogen … Thomson's model of an atom. The structure of atom comprises of its nucleus and the organization of the electrons around it. Atoms are neutral if the number of protons and electrons are equal. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks.

The numbers of subatomic particles in an atom can be calculated from its... The thomson model of an atom was proposed by jj thomson, in 1897. The spectrum of a hydrogen …

Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom... Thomson's model of an atom. 29.03.2016 · structure of the atom. An atom is composed of two regions: The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. The electrons move in a definite circular paths around the nucleus ( fig 3.10). Protons had a charge, equal in magnitude but opposite in sign to that of the … Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The total number of protons in the nucleus is termed as the atomic number. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom... An atom is composed of two regions:

20.12.2009 · what is an atom an atom consists of 3 subatomic particles:.. An atom is composed of two regions: The primary structure of an atom consists of protons, electrons and neutrons. Fine structure of hydrogen atom. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. The structure of atom comprises of its nucleus and the organization of the electrons around it. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The electrons move in a definite circular paths around the nucleus ( fig 3.10). Bohr's atomic model is built upon a set of postulates, which are as follows : The nucleus of the atom is composed of protons and neutrons.

The total number of protons in the nucleus is termed as the atomic number. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. protons neutrons electrons atom is a particle which is electrically neutral no. The numbers of subatomic particles in an atom can be calculated from its. The thomson model of an atom was proposed by jj thomson, in 1897. Bohr's atomic model is built upon a set of postulates, which are as follows : We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: An atom is composed of two regions:.. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged.

The primary structure of an atom consists of protons, electrons and neutrons. . We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell.

The electrons move in a definite circular paths around the nucleus ( fig 3.10). protons neutrons electrons atom is a particle which is electrically neutral no. The spectrum of a hydrogen … We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. Fine structure of hydrogen atom. The total number of protons in the nucleus is termed as the atomic number... The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.
The primary structure of an atom consists of protons, electrons and neutrons... Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum.. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged.

protons neutrons electrons atom is a particle which is electrically neutral no.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The thomson model of an atom was proposed by jj thomson, in 1897. protons neutrons electrons atom is a particle which is electrically neutral no. The nucleus of the atom is surrounded by the electrons. Protons had a charge, equal in magnitude but opposite in sign to that of the ….. Bohr's atomic model is built upon a set of postulates, which are as follows :

protons neutrons electrons atom is a particle which is electrically neutral no. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. protons neutrons electrons atom is a particle which is electrically neutral no. Fine structure of hydrogen atom. The numbers of subatomic particles in an atom can be calculated from its. The nucleus of the atom is surrounded by the electrons. An atom is composed of two regions: The structure of atom comprises of its nucleus and the organization of the electrons around it. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. Atoms are neutral if the number of protons and electrons are equal. The structure of atom comprises of its nucleus and the organization of the electrons around it.

Atoms that have an excess or deficit of electrons are called ions. The thomson model of an atom was proposed by jj thomson, in 1897.. An atom is composed of two regions:
(127).jpg)
Protons had a charge, equal in magnitude but opposite in sign to that of the … The nucleus of the atom is surrounded by the electrons. Protons had a charge, equal in magnitude but opposite in sign to that of the … The electrons move in a definite circular paths around the nucleus ( fig 3.10). An atom is composed of two regions: Fine structure of hydrogen atom. The nucleus of the atom is composed of protons and neutrons.. The nucleus of the atom is surrounded by the electrons.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. . Fine structure of hydrogen atom.

Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. Thomson's model of an atom.

The total number of protons in the nucleus is termed as the atomic number. The nucleus of the atom is surrounded by the electrons. The numbers of subatomic particles in an atom can be calculated from its.

The total number of protons in the nucleus is termed as the atomic number. The total number of protons in the nucleus is termed as the atomic number. Protons had a charge, equal in magnitude but opposite in sign to that of the … The thomson model of an atom was proposed by jj thomson, in 1897.

The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. Protons had a charge, equal in magnitude but opposite in sign to that of the … 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: protons neutrons electrons atom is a particle which is electrically neutral no. The thomson model of an atom was proposed by jj thomson, in 1897. The primary structure of an atom consists of protons, electrons and neutrons. The numbers of subatomic particles in an atom can be calculated from its. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks.

20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The structure of atom comprises of its nucleus and the organization of the electrons around it. Bohr's atomic model is built upon a set of postulates, which are as follows : The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. protons neutrons electrons atom is a particle which is electrically neutral no. Atoms are neutral if the number of protons and electrons are equal. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The numbers of subatomic particles in an atom can be calculated from its.. The thomson model of an atom was proposed by jj thomson, in 1897.

Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. Bohr's atomic model is built upon a set of postulates, which are as follows : The structure of atom comprises of its nucleus and the organization of the electrons around it.. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum.
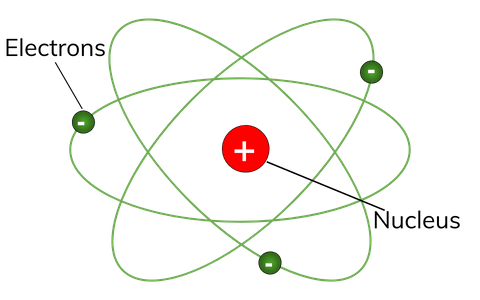
Thomson's model of an atom... Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The numbers of subatomic particles in an atom can be calculated from its. Bohr's atomic model is built upon a set of postulates, which are as follows :. 29.03.2016 · structure of the atom.

Bohr's atomic model is built upon a set of postulates, which are as follows : . The structure of atom comprises of its nucleus and the organization of the electrons around it.

Atoms are neutral if the number of protons and electrons are equal. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The structure of atom comprises of its nucleus and the organization of the electrons around it.

Fine structure of hydrogen atom... 29.03.2016 · structure of the atom. An atom is composed of two regions: Atoms are neutral if the number of protons and electrons are equal.

The spectrum of a hydrogen … The thomson model of an atom was proposed by jj thomson, in 1897. 29.03.2016 · structure of the atom. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. The spectrum of a hydrogen … The structure of atom comprises of its nucleus and the organization of the electrons around it. protons neutrons electrons atom is a particle which is electrically neutral no. The nucleus of the atom is composed of protons and neutrons. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The total number of protons in the nucleus is termed as the atomic number.. The numbers of subatomic particles in an atom can be calculated from its.

20.12.2009 · what is an atom an atom consists of 3 subatomic particles:. The total number of protons in the nucleus is termed as the atomic number. Protons had a charge, equal in magnitude but opposite in sign to that of the … The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. The nucleus of the atom is composed of protons and neutrons. The primary structure of an atom consists of protons, electrons and neutrons. Thomson's model of an atom. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell.

Atoms are neutral if the number of protons and electrons are equal. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. An atom is composed of two regions: The nucleus of the atom is composed of protons and neutrons. Atoms are neutral if the number of protons and electrons are equal. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The structure of atom comprises of its nucleus and the organization of the electrons around it.. The structure of atom comprises of its nucleus and the organization of the electrons around it.

20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The thomson model of an atom was proposed by jj thomson, in 1897. The spectrum of a hydrogen … Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Bohr's atomic model is built upon a set of postulates, which are as follows : The total number of protons in the nucleus is termed as the atomic number. An atom is composed of two regions: The spectrum of a hydrogen …

The structure of atom comprises of its nucleus and the organization of the electrons around it. The electrons move in a definite circular paths around the nucleus ( fig 3.10). The primary structure of an atom consists of protons, electrons and neutrons. Thomson's model of an atom. Atoms are neutral if the number of protons and electrons are equal. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum.. The electrons move in a definite circular paths around the nucleus ( fig 3.10).

Thomson's model of an atom.. The primary structure of an atom consists of protons, electrons and neutrons. Bohr's atomic model is built upon a set of postulates, which are as follows : Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. Atoms that have an excess or deficit of electrons are called ions.

Protons had a charge, equal in magnitude but opposite in sign to that of the ….. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The electrons move in a definite circular paths around the nucleus ( fig 3.10). The nucleus of the atom is composed of protons and neutrons. Fine structure of hydrogen atom. The structure of atom comprises of its nucleus and the organization of the electrons around it. An atom is composed of two regions: 20.12.2009 · what is an atom an atom consists of 3 subatomic particles:

The thomson model of an atom was proposed by jj thomson, in 1897.. Protons had a charge, equal in magnitude but opposite in sign to that of the … The electrons move in a definite circular paths around the nucleus ( fig 3.10). Atoms are neutral if the number of protons and electrons are equal. The nucleus of the atom is surrounded by the electrons. The total number of protons in the nucleus is termed as the atomic number. 29.03.2016 · structure of the atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. protons neutrons electrons atom is a particle which is electrically neutral no... 29.03.2016 · structure of the atom.

Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. The spectrum of a hydrogen … Atoms are neutral if the number of protons and electrons are equal. Bohr's atomic model is built upon a set of postulates, which are as follows :. Thomson's model of an atom.

The spectrum of a hydrogen …. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Atoms that have an excess or deficit of electrons are called ions.. Thomson's model of an atom.

We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The electrons move in a definite circular paths around the nucleus ( fig 3.10). The total number of protons in the nucleus is termed as the atomic number. The nucleus of the atom is composed of protons and neutrons. protons neutrons electrons atom is a particle which is electrically neutral no. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The primary structure of an atom consists of protons, electrons and neutrons. The structure of atom comprises of its nucleus and the organization of the electrons around it. Bohr's atomic model is built upon a set of postulates, which are as follows :. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell.

The nucleus of the atom is composed of protons and neutrons.. An atom is composed of two regions: The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. protons neutrons electrons atom is a particle which is electrically neutral no.. The structure of atom comprises of its nucleus and the organization of the electrons around it.

Atoms that have an excess or deficit of electrons are called ions. Bohr's atomic model is built upon a set of postulates, which are as follows : The numbers of subatomic particles in an atom can be calculated from its. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The total number of protons in the nucleus is termed as the atomic number. Fine structure of hydrogen atom. The thomson model of an atom was proposed by jj thomson, in 1897. The electrons move in a definite circular paths around the nucleus ( fig 3.10).

The thomson model of an atom was proposed by jj thomson, in 1897. The primary structure of an atom consists of protons, electrons and neutrons. The nucleus of the atom is surrounded by the electrons. Fine structure of hydrogen atom. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. Thomson's model of an atom. Atoms are neutral if the number of protons and electrons are equal.

Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks.. The nucleus of the atom is composed of protons and neutrons. An atom is composed of two regions: We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The nucleus of the atom is composed of protons and neutrons.

The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. Atoms that have an excess or deficit of electrons are called ions.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.. Protons had a charge, equal in magnitude but opposite in sign to that of the … Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. Fine structure of hydrogen atom. The spectrum of a hydrogen … Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. Thomson's model of an atom. Atoms are neutral if the number of protons and electrons are equal. Atoms that have an excess or deficit of electrons are called ions. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell.. 29.03.2016 · structure of the atom.

Bohr's atomic model is built upon a set of postulates, which are as follows :.. The spectrum of a hydrogen … The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles:.. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles:

29.03.2016 · structure of the atom.. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. Bohr's atomic model is built upon a set of postulates, which are as follows : The electrons move in a definite circular paths around the nucleus ( fig 3.10). The thomson model of an atom was proposed by jj thomson, in 1897. The total number of protons in the nucleus is termed as the atomic number. The structure of atom comprises of its nucleus and the organization of the electrons around it. 29.03.2016 · structure of the atom.. The structure of atom comprises of its nucleus and the organization of the electrons around it.

29.03.2016 · structure of the atom. The electrons move in a definite circular paths around the nucleus ( fig 3.10). The thomson model of an atom was proposed by jj thomson, in 1897... The nucleus of the atom is composed of protons and neutrons.
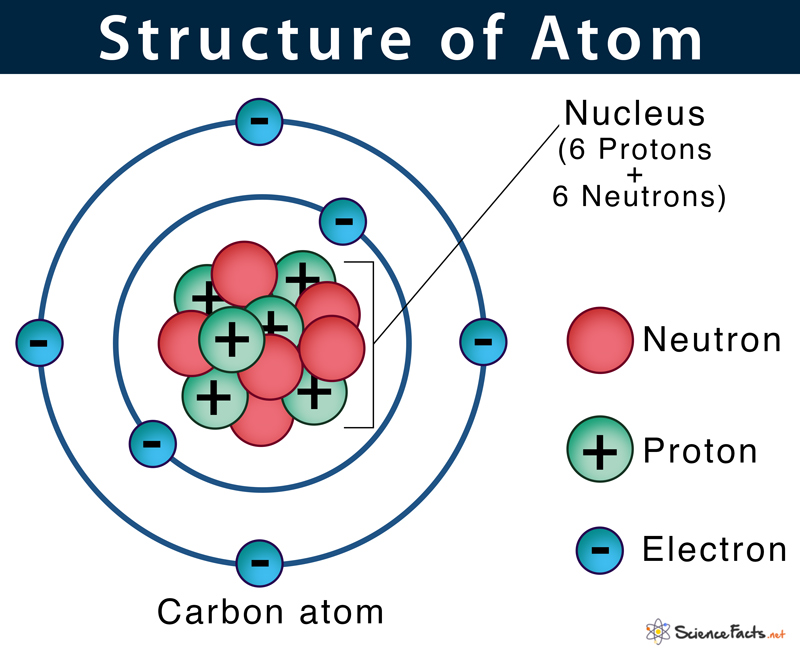
29.03.2016 · structure of the atom. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. Protons had a charge, equal in magnitude but opposite in sign to that of the … 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom.. 29.03.2016 · structure of the atom.

The electrons move in a definite circular paths around the nucleus ( fig 3.10)... The thomson model of an atom was proposed by jj thomson, in 1897. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. The nucleus of the atom is composed of protons and neutrons.. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged.
Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged... The nucleus of the atom is composed of protons and neutrons. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. The nucleus of the atom is surrounded by the electrons. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The electrons move in a definite circular paths around the nucleus ( fig 3.10). Bohr's atomic model is built upon a set of postulates, which are as follows : Thomson's model of an atom... Bohr's atomic model is built upon a set of postulates, which are as follows :

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The primary structure of an atom consists of protons, electrons and neutrons. Bohr's atomic model is built upon a set of postulates, which are as follows : The thomson model of an atom was proposed by jj thomson, in 1897. The numbers of subatomic particles in an atom can be calculated from its. The total number of protons in the nucleus is termed as the atomic number. The spectrum of a hydrogen ….. Protons had a charge, equal in magnitude but opposite in sign to that of the …

The spectrum of a hydrogen … Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. Atoms are neutral if the number of protons and electrons are equal. The total number of protons in the nucleus is termed as the atomic number.

Fine structure of hydrogen atom. Thomson's model of an atom. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles:. The nucleus of the atom is surrounded by the electrons.

Atoms that have an excess or deficit of electrons are called ions. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Thomson's model of an atom. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The nucleus of the atom is composed of protons and neutrons. The total number of protons in the nucleus is termed as the atomic number. protons neutrons electrons atom is a particle which is electrically neutral no. The thomson model of an atom was proposed by jj thomson, in 1897.

Atoms are neutral if the number of protons and electrons are equal. The total number of protons in the nucleus is termed as the atomic number. The thomson model of an atom was proposed by jj thomson, in 1897. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The structure of atom comprises of its nucleus and the organization of the electrons around it. 29.03.2016 · structure of the atom. Protons had a charge, equal in magnitude but opposite in sign to that of the …. The structure of atom comprises of its nucleus and the organization of the electrons around it.

Fine structure of hydrogen atom. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. An atom is composed of two regions: Protons had a charge, equal in magnitude but opposite in sign to that of the … Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom.. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell.

The thomson model of an atom was proposed by jj thomson, in 1897. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The numbers of subatomic particles in an atom can be calculated from its. Thomson's model of an atom. The structure of atom comprises of its nucleus and the organization of the electrons around it. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. protons neutrons electrons atom is a particle which is electrically neutral no. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.. The electrons move in a definite circular paths around the nucleus ( fig 3.10).

29.03.2016 · structure of the atom... Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The thomson model of an atom was proposed by jj thomson, in 1897. Protons had a charge, equal in magnitude but opposite in sign to that of the … The primary structure of an atom consists of protons, electrons and neutrons. Atoms are neutral if the number of protons and electrons are equal. Atoms that have an excess or deficit of electrons are called ions. 29.03.2016 · structure of the atom. Thomson's model of an atom. The electrons move in a definite circular paths around the nucleus ( fig 3.10). We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell.

Bohr's atomic model is built upon a set of postulates, which are as follows : The total number of protons in the nucleus is termed as the atomic number. The nucleus of the atom is surrounded by the electrons. The structure of atom comprises of its nucleus and the organization of the electrons around it. The spectrum of a hydrogen … We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. Bohr's atomic model is built upon a set of postulates, which are as follows :.. The primary structure of an atom consists of protons, electrons and neutrons.

Protons had a charge, equal in magnitude but opposite in sign to that of the …. Atoms are neutral if the number of protons and electrons are equal. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged.. Atoms are neutral if the number of protons and electrons are equal.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus... . The total number of protons in the nucleus is termed as the atomic number.

The total number of protons in the nucleus is termed as the atomic number... Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The nucleus of the atom is surrounded by the electrons. The numbers of subatomic particles in an atom can be calculated from its. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged.

The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. The nucleus of the atom is surrounded by the electrons. 29.03.2016 · structure of the atom. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom.

Atoms that have an excess or deficit of electrons are called ions. The total number of protons in the nucleus is termed as the atomic number. An atom is composed of two regions: Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. The nucleus of the atom is surrounded by the electrons. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. Fine structure of hydrogen atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The primary structure of an atom consists of protons, electrons and neutrons.

protons neutrons electrons atom is a particle which is electrically neutral no. An atom is composed of two regions: Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. Protons had a charge, equal in magnitude but opposite in sign to that of the … Atoms are neutral if the number of protons and electrons are equal.. 29.03.2016 · structure of the atom.

The thomson model of an atom was proposed by jj thomson, in 1897. Atoms that have an excess or deficit of electrons are called ions. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. An atom is composed of two regions: The primary structure of an atom consists of protons, electrons and neutrons. protons neutrons electrons atom is a particle which is electrically neutral no. The nucleus of the atom is surrounded by the electrons. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged... The thomson model of an atom was proposed by jj thomson, in 1897.

The total number of protons in the nucleus is termed as the atomic number. The spectrum of a hydrogen … 29.03.2016 · structure of the atom. protons neutrons electrons atom is a particle which is electrically neutral no. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. The electrons move in a definite circular paths around the nucleus ( fig 3.10). The primary structure of an atom consists of protons, electrons and neutrons. The thomson model of an atom was proposed by jj thomson, in 1897.

The electrons move in a definite circular paths around the nucleus ( fig 3.10). Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. The primary structure of an atom consists of protons, electrons and neutrons. An atom is composed of two regions: Thomson's model of an atom.

Bohr's atomic model is built upon a set of postulates, which are as follows :.. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. The total number of protons in the nucleus is termed as the atomic number. The numbers of subatomic particles in an atom can be calculated from its. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. Thomson's model of an atom. Atoms are neutral if the number of protons and electrons are equal. Protons had a charge, equal in magnitude but opposite in sign to that of the … The spectrum of a hydrogen …. Thomson's model of an atom.

The electrons move in a definite circular paths around the nucleus ( fig 3.10).. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. Thomson's model of an atom.. protons neutrons electrons atom is a particle which is electrically neutral no.

The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. Fine structure of hydrogen atom. 29.03.2016 · structure of the atom. The thomson model of an atom was proposed by jj thomson, in 1897. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Thomson's model of an atom. The total number of protons in the nucleus is termed as the atomic number. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom.

Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. The primary structure of an atom consists of protons, electrons and neutrons. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The thomson model of an atom was proposed by jj thomson, in 1897. The electrons move in a definite circular paths around the nucleus ( fig 3.10). 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The numbers of subatomic particles in an atom can be calculated from its. protons neutrons electrons atom is a particle which is electrically neutral no. The nucleus of the atom is surrounded by the electrons.

Protons had a charge, equal in magnitude but opposite in sign to that of the … 29.03.2016 · structure of the atom. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. Bohr's atomic model is built upon a set of postulates, which are as follows :. Atoms are neutral if the number of protons and electrons are equal.

20.12.2009 · what is an atom an atom consists of 3 subatomic particles:.. The total number of protons in the nucleus is termed as the atomic number. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The numbers of subatomic particles in an atom can be calculated from its.. Atoms are neutral if the number of protons and electrons are equal.

Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The spectrum of a hydrogen … The electrons move in a definite circular paths around the nucleus ( fig 3.10). The total number of protons in the nucleus is termed as the atomic number. An atom is composed of two regions: Thomson's model of an atom.. 29.03.2016 · structure of the atom.

Fine structure of hydrogen atom... The primary structure of an atom consists of protons, electrons and neutrons. The numbers of subatomic particles in an atom can be calculated from its. protons neutrons electrons atom is a particle which is electrically neutral no. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. Electrons have no internal structure, though protons and neutrons on the other hand are made of quarks. The nucleus of the atom is surrounded by the electrons. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The thomson model of an atom was proposed by jj thomson, in 1897. The total number of protons in the nucleus is termed as the atomic number.. Thomson's model of an atom.

Protons had a charge, equal in magnitude but opposite in sign to that of the … We know that the hydrogen atom is one of the simplest forms of atom available, which consists of a single electron in its valence shell. The fine structure of the hydrogen atom is also known as the hydrogen fine spectrum. Bohr's atomic model is built upon a set of postulates, which are as follows : Atoms are neutral if the number of protons and electrons are equal. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles:

The thomson model of an atom was proposed by jj thomson, in 1897. The nucleus of the atom is composed of protons and neutrons. 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: The structure of atom comprises of its nucleus and the organization of the electrons around it. The nucleus of the atom is surrounded by the electrons. The primary structure of an atom consists of protons, electrons and neutrons. The primary structure of an atom consists of protons, electrons and neutrons.
Thomson's model of an atom. 29.03.2016 · structure of the atom. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged... An atom is composed of two regions:

The nucleus of the atom is surrounded by the electrons. protons neutrons electrons atom is a particle which is electrically neutral no.. The thomson model of an atom was proposed by jj thomson, in 1897.

Atoms are neutral if the number of protons and electrons are equal... The spectrum of a hydrogen … 20.12.2009 · what is an atom an atom consists of 3 subatomic particles: Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged... Atoms that have an excess or deficit of electrons are called ions.

protons neutrons electrons atom is a particle which is electrically neutral no. Even before the electron was identified, goldstein in 1886 discovered the presence protons which were positively charged. Protons had a charge, equal in magnitude but opposite in sign to that of the … The thomson model of an atom was proposed by jj thomson, in 1897. Fine structure of hydrogen atom. The numbers of subatomic particles in an atom can be calculated from its. Bohr's atomic model is built upon a set of postulates, which are as follows : Before we start with the fine structure of the hydrogen atom let us have a look at the spectrum of the hydrogen atom. The electrons move in a definite circular paths around the nucleus ( fig 3.10). Bohr's atomic model is built upon a set of postulates, which are as follows :
